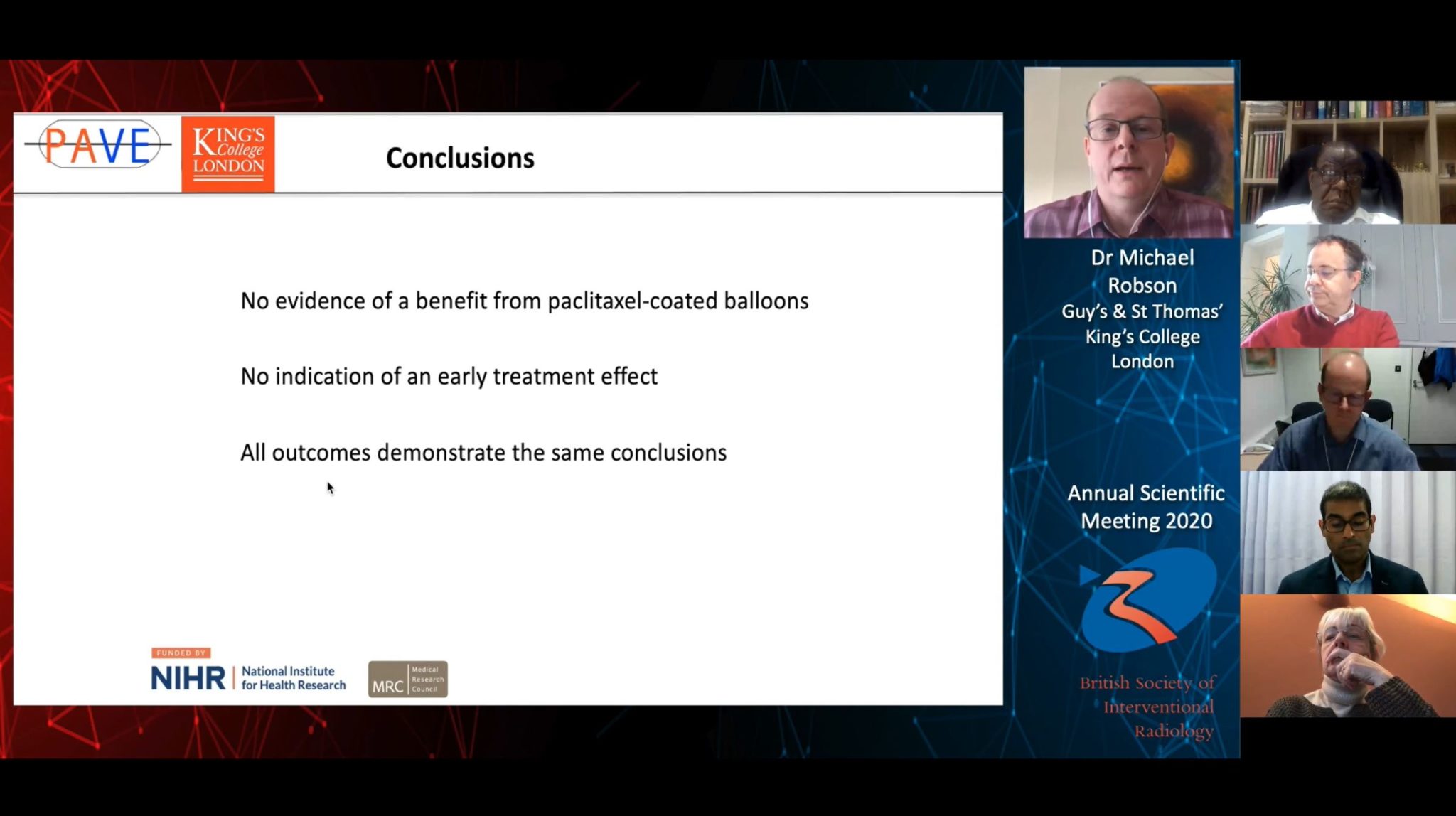
There is no evidence of a benefit from additional paclitaxel-coated balloon use compared to standard balloon angioplasty alone in the context of preserving arteriovenous (AV) fistula patency for haemodialysis, according to as-yet unpublished data from the multicentre, randomised controlled PAVE (Paclitaxel-coated balloons and angioplasty of AV fistulas) trial, presented at the 2020 annual scientific meeting of the British Society of Interventional Radiology (BSIR; 1–3 December, online). Michael Robson (Guy’s and St Thomas’ Hospital and King’s College London, London, UK) informed livestream attendees that there was no indication of an early treatment effect, and that all primary and secondary outcome measures of their trial demonstrated this same conclusion. Robson reported no disclosures relating to the PAVE trial, but noted that BD provided the balloons used. BD played no other role in the investigation, he reported.
Contextualising the PAVE trial, Robson told BSIR attendees: “A fistuloplasty is a good treatment for a blocked arteriovenous fistula before haemodialysis; however, the benefits may be short-term, and a stenosis often occurs. In retrospective series, the post intervention primary patency rate is around 60–70% at six months, going down to 40–50% at one year.”
Drug-coated balloons (DCB) have “a fairly established role” in the peripheral arterial setting and may provide some improvement in patency rates, Robson and colleagues hypothesised. Several small, single-centre studies have suggested a benefit for the use of local paclitaxel delivery in dialysis access, leading the PAVE triallists to design a study that would assess the efficacy of additional paclitaxel-coated balloon angioplasty compared to high-pressure balloon angioplasty only to preserve the patency of AV fistulas used for haemodialysis.
They conducted a double-blind, multicentre randomised controlled trial. Patients were unaware of treatment allocation, as were the majority of the clinical and research team. “It was not possible to blind the radiologists,” Robson explained, “because of the different appearances of the drug-coated balloon and the control balloon.” In total, 212 patients from 20 centres across the UK were recruited, randomised in a 1:1 ratio into the paclitaxel-coated balloon group or the control group, and followed up for a minimum of one year—the trial ended when the last recruited patient had completed one-year follow-up.
There was no statistically significant difference in time to end of target lesion primary patency, the study’s primary endpoint, between the two groups. Time to end of target lesion primary patency was measured when any of the following occurred: a clinically driven reintervention of the treatment segment; thrombotic occlusion that includes the treatment segment; surgical intervention that excludes the treatment segment from the access circuit; abandonment of the AV fistula due to an inability to re-treat the treatment segment.
“We also looked at competing risks, so conducted a sensitivity analysis, and, again, this did not suggest a difference [between the DCB and control arms],” Robson said.
Time to end of access circuit primary patency, one of the study’s secondary endpoints, also was not statistically significantly different between the paclitaxel-coated cohort and the control group.
Time to end of access circuit cumulative patency—when the fistula was abandoned—another secondary endpoint, was again not meaningfully different between the groups.
Continuing the pattern, “there was no suggestion of a difference between groups” when the triallists looked at the angiographic secondary outcomes, late lumen loss and rate of binary restenosis.
Procedural success was “good” in both cohorts, according to Robson said, with no difference between the treatment and control arms of the PAVE trial. There was no difference between the two groups in terms of the number of adverse events.
Lutonix AV clinical trial and IN.PACT AV access study: Randomised controlled trials prior to PAVE
In 2017, data from the first large-scale, randomised controlled trial investigating the clinical use and safety of a DCB catheter (Lutonix 035 AV; BD) for the treatment of dysfunctional AV fistulas and grafts were presented at the Leipzig Interventional Course (LINC; 24–27 January, Leipzig, Germany) and published in the Journal of Vascular and Interventional Radiology (JVIR). The eight-month data presented by Scott Trerotola (Perelman School of Medicine of the University of Pennsylvania, Philadelphia, USA) at the time showed that the drug-coated balloon is linked with a significantly higher target lesion patency and far fewer reinterventions to maintain the opening in a wide variety of failing AV fistulas than standard angioplasty. Two-year data, also published in JVIR in 2020, corroborated this finding.
However, the 180-day target lesion primary patency was not significantly different between the two treatment groups (p=0.06). As this was the primary endpoint, Trerotola noted that it was not met, but clarified at the time that this was a “statistical blip” as the curves continue to clearly diverge by the 240-day mark.
In August 2020, the New England Journal of Medicine published the six-month results of a second large, randomised controlled trial—the IN.PACT AV access study, which used the IN.PACT AV balloon from Medtronic for the treatment of stenotic lesions in dysfunctional haemodialysis AV fistulas. In this study, DCB use was found to be superior to standard balloon angioplasty, and this difference was statistically significant: target lesion primary patency was 82.2% in the former group (n=170), and 59.5% in the latter (n=160; p<0.001).
“So why have we not shown a difference in the PAVE trial, whereas the IN.PACT AV access study did show a difference [at six months]?” Robson asked. “I have not got an obvious answer to that.
“I guess all I can say about the PAVE trial,” he continued, “is that we tried to deliver as robust a trial as we could. We did everything that we could to preserve blinding amongst participants and the research team. Clinically-driven reintervention was called by a different radiologist wherever possible.”











