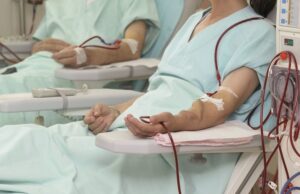
Femoral vein transposition AVF a “feasible and realiable” option in complex patients
Femoral vein transposition arteriovenous fistula (FV tAVF) has been shown to be a “feasible and reliable” option for haemodialysis vascular access in complex patients,...
Post-approval study of EndoForce anastomotic connector commences with first cases
Phraxis has announced the successful initiation of its post-approval study (PAS) for the EndoForce anastomotic connector, with the first procedure performed at Spartanburg Regional...
Drug trial shows promise in treating FSGS
Boehringer Ingelheim has announced results from a 12-week phase II clinical trial evaluating apecotrep, an oral, non-immunosuppressive TRPC6 inhibitor for people with primary focal...
“Plant-forward” diet may reduce risk of chronic kidney disease
Eating a “plant-forward” diet and limiting added sugars and fats as part of the EAT–Lancet planetary diet was associated with a reduced risk of...