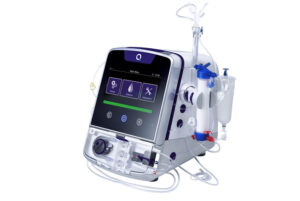
Quanta Dialysis Technologies has confirmed the US Food and Drug Administration (FDA) has given 510(k) clearance for the use of its Quanta dialysis system in the home setting. With this clearance, Quanta states that they have become the only company to offer a high dialysate flow (500 mL/min) system across the entire care continuum for end stage renal disease (ESRD) patients.
This significant step in Quanta’s US commercialisation efforts follows another recent milestone in which the company received clearance for the first, and only, US FDA-cleared device able to perform intermittent haemodialysis (IHD), sustained low efficiency dialysis (SLED), and continuous renal replacement therapy (CRRT) continuous venovenous haemodialysis [CVVHD] and slow continuous ultrafiltration [SCUF]) without any need for bags.
Based on 2022 data from the United States Renal Data System, of the 550,000 prevalent ESRD patients in the country on dialysis therapy, just 2.4% receive home haemodialysis (HHD). The Quanta Dialysis System (previously known as the SC+ Haemodialysis System) addresses this gap by providing patients access to a high-flow system in the home, the same offering as routinely provided in hospitals, post-acute facilities and in-centre clinics.
“The 510(k) clearance embodies the hard work and determination of our exceptional team at Quanta,” said Quanta chief executive officer (CEO) Alejandro Galindo. “We recruited and executed this pivotal trial during the height of COVID-19 and have now achieved an US FDA clearance few other products have obtained. As the USA continues to evolve towards value-based care models, the focus for patients with chronic diseases is on minimising complications and re-admissions.
“Our product is designed to help those patients seamlessly transition to the home and remain there as long as possible. We are currently planning our home launch with customers who have successfully implemented Quanta into acute and sub-acute settings first.”
Decades-Long Troubles with HHD Adoption
Across the USA, more than 70% of dialysis facilities are currently not certified to offer HHD, and almost half of those with certification have no active HHD patients. Much of this is attributed to the lack of US FDA-cleared technologies, alongside challenges associated with the cost-of-therapy and patient ‘burnout’, caused by dialysing with low flow technologies requiring frequent treatments.
“Many patients prefer the flexibility of home haemodialysis—a flexible schedule, no commute, and with more frequent therapies they feel better and need less medication,” said Quanta chief medical officer (CMO) Paul Komenda. “Expanding treatment into the home is something we are constantly asked about by clinicians, advocacy groups, providers and patients. One of our major commitments as a business is to make kidney care more accessible, and with an easy-to-train, easy-to-maintain device that accommodates a high-flow HD prescription, Quanta is poised to become the best option for the home.”
Encouraging Clinical Trial Results
A US FDA Investigational Device Exemption (IDE) trial of the Quanta dialysis system was completed in October 2023, including a multi-centre, open-label assessment of efficacy and safety. The trial saw 32 patients receive standard in-centre haemodialysis while training to use the Quanta dialysis system, before transitioning to perform HHD four times per week for eight weeks. The study demonstrated the device to be safe and effective, with more than 90% of patients electing to continue using the device after completing the trial.
Study results were presented at the American Society of Nephrology meeting in 2023 and the manuscript is now under review at the Clinical Journal of the American Society of Nephrology.
“Our IDE study has clearly demonstrated best in class dialysis adequacy and high patient satisfaction. Our device gives sufficient dialysis in three times per week therapy and the option of better outcomes with higher frequency,” added Komenda.











