 Vascular Flow Technologies has announced that it has received US Food and Drug Administration (FDA) clearance for its Spiral Laminar Flow arteriovenous (AV) graft portfolio.
Vascular Flow Technologies has announced that it has received US Food and Drug Administration (FDA) clearance for its Spiral Laminar Flow arteriovenous (AV) graft portfolio.
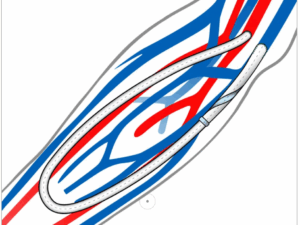
The SLF AV graft is an ePTFE haemodialysis graft with a unique design feature on the venous end of the graft, which remodels the blood flow in a spiral laminar fashion.
The SLF AV Graft benefits patients by decreasing turbulent blood flow at the venous anastomosis, improving overall patency, while slowing the progression of venous neointimal hyperplasia (VNIH), which accounts for the overwhelming majority of ePTFE AV Graft failure, the company said in a press release.
“We are excited to bring a novel and innovative AV graft to the US market,” said Craig Dunlop, general manager of Vascular Flow, “This technology will be of great long-term benefit to people suffering from end-stage renal disease.”