Tag: Medtronic
CMS grants transitional pass-through payment for Medtronic’s Symplicity Spyral and Recor’s...
The Centers for Medicare and Medicaid Services (CMS) has granted transitional pass-through (TPT) payment for Medtronic's Symplicity Spyral renal denervation (RDN) catheter and Recor...
IN.PACT AV Access five-year results strengthen physicians’ conviction on applying DCB...
Alexander Mallios (Paris, France) and Andrew Holden (Auckland, New Zealand; one of the co-chairs of the Charing Cross Symposium) share their views on...
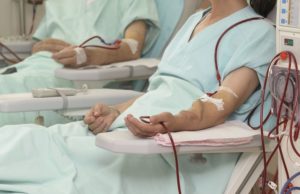
US survey shows lack of kidney patient awareness of minimally invasive...
A survey recently conducted by Medtronic and the US National Kidney Foundation (NKF) found that patients undergoing haemodialysis lack awareness about minimally invasive treatment...
Amazon executive joins Medtronic to spearhead development in robotics and implantables
Medtronic has announced that Ken Washington has been appointed chief technology and innovation officer.
Washington joins Medtronic from Amazon where he served as vice president...
Medtronic, DaVita launch Mozarc Medical, a new company aimed at accelerating...
Medtronic and DaVita have announced the launch of Mozarc Medical—an independent new company that, they state in a press release, is “committed to reshaping...
Roundtable: Will latest IN.PACT AV Access data impact practice and can...
NOTE: This video is ONLY available to watch in selected countries and geographies
In this Renal Interventions roundtable, Andrew Holden (moderator; Auckland, New Zealand) is...
SPYRAL HTN-ON MED study demonstrates meaningful clinical benefits consistent with other...
Medtronic has announced the six-month results from the full cohort of the SPYRAL HTN-ON MED clinical trial. The late-breaking data were presented at the...
History warns us against “optimistic exuberance” in drug-coated balloon trials
The importance of assessing clinical trial data in an objective, balanced way has been borne out by recent history in the vascular surgery space,...
Vascular access creation: EndoAVF keeps future options open, but is not...
Based on over four years of experience in a single centre, Robert Shahverdyan (Asklepios Klinik Barmbek, Hamburg, Germany) claims that endovascular arteriovenous fistula (endoAVF)...
Medtronic and DaVita agree to launch new kidney health technology company
Medtronic and DaVita have together announced their intent to form a new, independent kidney care-focused medical device company in an effort to enhance the patient...
First time data release: View the full IN.PACT AV Access trial...
NOTE: This video is ONLY available to watch in selected countries and geographies
In a late-breaking Podium 1st presentation at the 2022 Charing Cross...
IN.PACT AV DCB “first and only” to show sustained and superior...
Medtronic has announced new randomised controlled data demonstrating the sustained and superior performance of the IN.PACT AV drug-coated balloon (DCB) compared to percutaneous transluminal...
Medtronic names Laura Mauri as new chief scientific, medical and regulatory...
Medtronic has announced that Laura Mauri has been appointed as the company’s chief scientific, medical and regulatory officer. This appointment adds to Mauri's prior responsibilities...
Medtronic issues voluntary recall for subset of IN.PACT Admiral and IN.PACT...
Medtronic recently voluntarily recalled a subset of its IN.PACT Admiral and IN.PACT AV paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheters due to the potential...
Study indicates lower wound infection and surgical reintervention rates in pAVF...
A single-centre, retrospective, comparative study, which was presented at the July 2021 Journal of Vascular Surgery (JVS) Journal Club (14 July, online), has found...
Benefits of percutaneous fistula creation further bolstered by five-year Ellipsys data
The Ellipsys vascular access system (Avenu Medical/Medtronic) can be used to easily and safely create durable percutaneous arteriovenous fistulas (pAVFs) for haemodialysis—with new, long-term...
Medtronic unveils data on hypertension treatment preferences, launches SPYRAL AFFIRM study
Medtronic today announced findings from a new study of patient preferences for the treatment of hypertension. The findings are set to be presented during...
Medtronic’s Hugo robotic-assisted surgery system receives European CE mark approval
Medtronic has announced receipt of a CE mark for its Hugo robotic-assisted surgery (RAS) system, authorising the sale of the system in Europe. This CE mark...
IN.PACT AV DCB sustained “superior” effectiveness in subgroups with high reintervention...
Several subgroups of patients treated with the IN.PACT AV drug-coated balloon (DCB; Medtronic) for arteriovenous fistulas (AVFs) demonstrated a “statistically significant” higher rate of...
Medtronic announces first patient procedure with Hugo robotic-assisted surgery system
Medtronic has announced the first patient procedure with the Hugo robotic-assisted surgery (RAS) system. The robotic prostatectomy was performed by urological surgeon Ruben Olivares (Clínica Santa...
More mixed results for DCBs in AV access maintenance as evidence...
Additional randomised controlled data regarding the effectiveness of drug-coated balloons (DCBs) in maintaining arteriovenous (AV) access offers new evidence, but it is freighted with...
EuroPCR 2021: Late-breaking data demonstrate long-term benefits of Medtronic radiofrequency renal...
Medtronic today announced new clinical data from the Global SYMPLICITY registry (GSR) indicating that renal denervation with the Medtronic Symplicity renal denervation system was...
Two-year IN.PACT AV Access results presented at CX 2021
Medtronic recently announced the safety and effectiveness results through 24 months for the IN.PACT AV Access clinical study. The data, which were presented virtually...
Medtronic launches IN.PACT AV DCB in Japan for patients undergoing haemodialysis
Medtronic has announced the launch of IN.PACT AV drug-coated balloon (DCB) in Japan. IN.PACT AV DCB is indicated for the treatment of obstructive lesions...