Tag: US FDA
US FDA announces plans to address medical device shortage risks
The US Food and Drug Administration (FDA) has issued a statement outlining measures to enhance protections against medical device shortages.
According to Michelle Tarver,...
US FDA issues first draft guidance for AI-based medical device development
The US Food and Drug Administration (FDA) has issued draft guidance that includes recommendations to support development and marketing of safe and effective artificial...
VEITH audience hears when RCTs are essential for novel vascular access...
As a part of a debate on the last day of the 2024 VEITHsymposium (19–23 November, New York, USA), Robert E Lee, a medical...
Vivance completes pre-pivotal trial of wearable dialysis device
Vivance has recently announced that the next trial phase of the wearable peritoneal dialysis (PD) device, Viva Kompact, has successfully concluded. The study was...
Revelation Biosciences announces US FDA acceptance of Gemini IND
Revelation Biosciences recently announced that the US Food and Drug Administration (FDA) has accepted its investigational new drug (IND) application for Gemini. The company referred...
SoniVie appoints veteran medtech executive Raymond W Cohen as chairman of...
SoniVie Ltd. announced today the appointment of Raymond W Cohen to serve as its chairman of the board of directors.
Cohen has served as the...
Healionics’ STARgraft receives US FDA Breakthrough Device designation
Healionics Corporation has today announced that its STARgraft arteriovenous graft—which it states is designed to provide a safer and more reliable means of bloodstream...
Xeltis announces US FDA Breakthrough Device Designation for aXess and first...
Xeltis has today announced that the Center for Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA) has granted aXess...
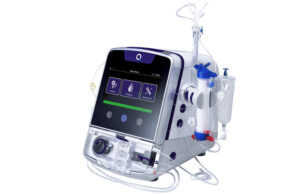
Quanta dialysis system receives US FDA clearance for home haemodialysis
Quanta Dialysis Technologies has confirmed the US Food and Drug Administration (FDA) has given 510(k) clearance for the use of its Quanta dialysis system...
Xeltis expands leadership team with appointment of Luc Verhees as vice...
Xeltis, a leading developer of transformative implants that enable the natural creation of living and long-lasting vessels, announces that it has appointed Luc Verhees...
Paragonix Technologies receives US FDA 510(k) clearance for KidneyVault portable renal...
Getinge’s most recent acquisition, Paragonix Technologies, has recently received US Food and Drug Administration (FDA) 510(k) clearance for its donor kidney preservation system, KidneyVault,...
VasQ extravascular support granted transitional pass-through (TPT) payment
Laminate Medical Technologies announced last month that the US Centers for Medicare & Medicaid Services (CMS) granted their VasQ extravascular support device a transitional pass-through (TPT)...
Akebia Therapeutics and U.S. Renal Care sign commercial supply contract to...
Akebia Therapeutics and U.S. Renal Care (USRC) have recently announced entry into a multi-year commercial supply contract encompassing all USRC dialysis centres. The contract...
VENOS-1 FIH trial one-year results demonstrate advantages of the next-generation Velocity...
This morning, at the Controversies in Dialysis Access (CiDA) 2024 meeting (3–5 October, Washington D.C., USA), Robert Shahverdyan (Hamburg, Germany) gave a presentation sharing...
Venova Medical announces US$30 million Series B financing and appointment of...
Venova Medical has announced the initial closing of a US$30M Series B funding round. Catalyst Health Ventures and a leading medical device company co-led...
Merit Medical to present six-month outcomes from randomised arm of WRAPSODY...
Merit Medical has announced today plans to release findings from its WAVE study. A pivotal, international, multicentre trial, the WAVE study examines the safety...
Diality joins Kidney Care partners
Diality has recently announced that it has joined Kidney Care Partners, the USAs leading kidney care multi-stakeholder coalition that represents patient advocates, physician organisations,...
Diality receives US FDA 510(k) clearance for its Moda-flx haemodialysis system
Diality announced today that it has secured 510(k) clearance from the US Food & Drug Administration (FDA) for its Moda-flx haemodialysis system. According to Diality’s...
SoniVie announces advancements in its renal denervation programme
SoniVie, which has developed a novel Therapeutic Intra-Vascular Ultrasound System (TIVUS) to treat a variety of hypertensive disorders, presented at the EuroPCR 2024 conference...
Xeltis appoints Shawn Gage as vice president of US clinical affairs
Xeltis has announced today the appointment of Shawn Gage as its new vice president of US clinical affairs. The company stated that his appointment...
Renalys Pharma announces first patient dosed in Phase III clinical trial...
Renalys Pharma has announced last week that the first person was dosed in its registrational Phase III clinical trial of sparsentan for the treatment...
Is renal denervation out of retirement?
“It’s rare to see a single trial kill something that was greeted with enthusiasm, so the question is why is renal denervation alive again?...
Renal denervation: Bridging specialty boundaries with better collaborations
Wei Li (Syracuse VA Medical Center, SUNY Upstate Medical University Syracuse, USA, and Texas Tech University School of Medicine, Lubbock, USA) and Aiyu Zhao...
Renal Interventions’ top 10 stories of February 2024
February was a month of trials beginning enrolment, novel technologies, and treatment protocols, with Gianmarco Falcone emphasising the need for a full protocol and...
US FDA grants 510(k) clearance to Fresenius Medical Care’s new haemodialysis...
Fresenius Medical Care recently announced via press release that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for its...
New research shows established immunosuppressant medications suitable for pig-to-human kidney transplants
Researchers at the University of Alabama at Birmingham (UAB) have discovered that immunosuppressant’s that are US Food and Drug Administration (FDA) approved—and that are...
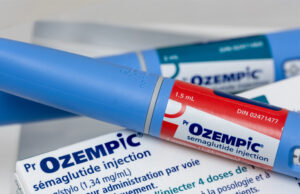
New option for chronic kidney failure patients emerges as Ozempic trial...
Ozempic (Novo Nordisk) is a once-daily injection with US Food and Drug Administration (FDA) approval for use as a preventative treatment for strokes and...
FDA removes red flag for paclitaxel-coated devices after review finds data...
In a letter to healthcare providers dated 11 July 2023, the US Food and Drug Administration (FDA) communicates that the risk of mortality associated...
Swiss parliament votes to accept US FDA-approved medical devices
The Swiss Federal Assembly has voted in favour of accepting medical devices with US Food and Drug Administration (FDA) marketing authorisation in Switzerland.
A motion...
FDA issues two final guidances for including patient perspectives in medical...
The US Food and Drug Administration (FDA) has issued two final guidances providing recommendations for including patient perspectives in medical device clinical studies.
As...
ImpediMed gains FDA Breakthrough Device designation for digital renal failure platform
ImpediMed, an Australia-based medical technology company using bioimpedance spectroscopy (BIS) to generate powerful data to improve patient health, has announced that the Sozo digital...
Fist Assist device for focal arm massage and increased vein circulation...
Fist Assist Devices has received US Food and Drug Administration (FDA) 510(k) clearance for use of the Fist Assist FA-1 device in the USA...