Venova Medical has announced the initial closing of a US$30M Series B funding round. Catalyst Health Ventures and a leading medical device company co-led the financing, with participation from ShangBay Capital, Mirae Asset Capital, KOFA Healthcare, Cadence Healthcare Ventures, Aphelion Capital and other new and existing investors.
Venova Medical has announced the initial closing of a US$30M Series B funding round. Catalyst Health Ventures and a leading medical device company co-led the financing, with participation from ShangBay Capital, Mirae Asset Capital, KOFA Healthcare, Cadence Healthcare Ventures, Aphelion Capital and other new and existing investors.
The funding will be used to further develop the company’s “next generation” technology for the creation of percutaneous arteriovenous fistulas (pAVF) for haemodialysis access and conduct clinical studies in the USA to support US Food and Drug Administration (FDA) regulatory approval. Venova Medical also announced the appointment of Darshana Zaveri, managing partner at Catalyst Health Ventures, to the company’s board of directors.
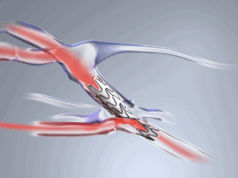
Venova Medical state that its Velocity pAVF system is an “innovative medical technology” that is being developed to use a minimally invasive technique to create an arteriovenous fistula (AVF) for haemodialysis vascular access. The Velocity pAVF system is designed to provide optimal and durable fistula flow to support maturation and clinical use for haemodialysis, using a percutaneous procedure that is intended for use in an office, surgery centre, or outpatient hospital setting.
“Providing a safe, effective and long-lasting method for vascular access is a significant challenge in haemodialysis treatment for patients with kidney failure,” said Zaveri. “Conventional methods such as surgical arteriovenous fistulas (AVF), arteriovenous grafts, or central venous catheters have not adequately met this need. Venova Medical’s technology aims to overcome the limitations of these traditional approaches, offering a transformative solution for patients dependent on haemodialysis”.
“Despite decades of tremendous efforts from healthcare providers, the vast majority of patients starting dialysis do so with a central venous catheter, subjecting them to increased risk of morbidity and mortality and cost to the U.S. healthcare system as compared to the safer AVF procedure,” stated Erik van der Burg, chief executive officer (CEO) of Venova Medical. “We believe that the Velocity pAVF System will become the new standard of care and reduce the barriers preventing a majority of patients from starting dialysis with an AVF.”