Phraxis has announced US Food and Drug Administration (FDA) approval of the EndoForce connector for endovascular venous anastomosis (EndoForce).
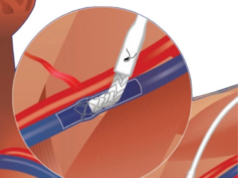
EndoForce is a patented endovascular implant designed to simplify and modernise the creation of arteriovenous grafts (AVGs) for haemodialysis, Phraxis says in a press release. The device enables a unique endovascular anastomosis, eliminating the need for surgical dissection of the venous anastomosis and promoting precise, coaxial vessel-to-graft alignment. EndoForce is engineered to reduce tissue trauma, streamline procedural workflow, and support long-term graft performance.
John Ross (Medical University of South Carolina, Orangeburg, USA) lead principal investigator for the pivotal study of the device, commented: “I’m excited to see the EndoForce provide a novel anastomotic option for AVG creation. This straightforward and innovative approach enhances procedural efficiency while addressing key challenges in vascular access. Its unique design has the potential to reduce complications such as intimal hyperplasia at the graft-to-vein anastomosis, ultimately supporting improved long-term outcomes for dialysis patients.”
The device is compatible with standard 6mm ePTFE AVGs and incorporates several proprietary elements: anchoring barbs that secure the device within the vein; a flexible, ePTFE-covered nitinol segment that conforms to the graft and vessel wall; and a compressible section that expands securely within the AVG upon deployment. This configuration supports a stable, end-to-end anastomosis intended to promote laminar blood flow and reduce turbulent shear stress—factors that contribute to endothelial buildup and graft failure.
In a pivotal, multicentre, single-arm study, EndoForce achieved procedural success, meeting its primary endpoint of cumulative patency at six months, with a 92% patency rate. Secondary endpoints—including primary patency and technical success—further reinforced the device’s strong clinical performance.
Alexander Yevzlin, chief executive officer of Phraxis, stated: “FDA approval of the EndoForce connector marks a major advancement for the dialysis community. The device introduces a new standard for AVG creation and has demonstrated exceptional ease of use and procedural reliability. While long-term outcomes are still being evaluated, we remain optimistic about its potential to improve dialysis care.”