This advertorial is sponsored by Laminate Medical Technologies
In medicine, the pursuit of better patient outcomes fuels the quest for innovation. Haemodialysis patients and vascular surgeons seeking consistent, patent vascular access have been discouraged by standard, unsupported arteriovenous fistulas (AVF) functional success rates ranging from 40–72% as reported in landmark studies over the years.1-8 These consistently high rates of failures were assumed to be a natural ceiling to functional success rooted in unavoidable mechanical and haemodynamic factors that arise when you arterialise a vein and lead to poor maturation.
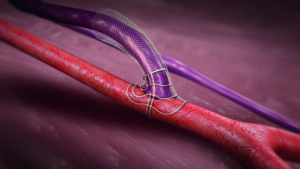
In response, Laminate Medical Technologies created a new device concept by which the surgeon can structurally reinforce the outflow vein using an external vascular support and guide its maturation in a more mechanically and haemodynamically stable configuration. That device, called VasQ™ Extravascular, was designed specifically to address these two factors of arteriovenous fistula failure that have plagued vascular access surgeons for decades. VasQ is a laser-cut nitinol brace that sits on the artery and sets the angle as well as the baseline diameter of the vein at the level of the anastomosis. The remaining ~2.5cm of vein is surrounded by a flexible woven nitinol brace that diametrically tapers outward, facilitating a gradual transition of a flow with a more laminar profile through the juxta-anastomotic segment to the outflow vein. To date, this structural reinforcement that guides a more optimal venous remodelling has led to functional success rates ranging between 88–100% across nine studies, as well as potential long-term benefits for surgical fistula outcomes by providing permanent reinforcement to the artery-vein connection.8-16
Here, five surgeons from across the world who have recently integrated VasQ into their practices offer their unique perspectives—Ari Kramer (Spartanburg Medical Center, Spartanburg, USA), George Blessios (Kaleida Health, Buffalo, USA), Paul Gibbs (Queen Alexander Hospital, Portsmouth, UK), and Yong Enming and Zhang Li (both Tan Tock Seng Hospital, Novena, Singapore). Many medical devices show promising clinical data but can fall short in real-world cases. However, following nearly a decade of work, leading surgeons from across the world agree, VasQ’s clinical data is overwhelmingly positive and adoption of VasQ has helped to consistently improve AVF outcomes as promised, positioning VasQ as a device with the potential to reshape the standard of care for haemodialysis patients worldwide.
Kramer – South Carolina, USA
“As the director and principal surgeon of Vascular Access Surgery at Spartanburg Regional Hospital, I was thrilled to be the first in the USA to implement VasQ after it was granted US Food and Drug Administration (FDA) de novo status, marking an exciting opportunity for patients seeking improved outcomes from surgical fistulas. Faced with the limitations of traditional methods, especially their disappointing maturation rates and particularly at the wrist, VasQ stood out as an innovative, low-risk solution promising to give my patients the best chance for a usable fistula without as many additional procedures. The transition to this technique was smooth, and its initial performance not only met but exceeded our high expectations, reflecting the device’s impressive clinical data. With VasQ, I’ve observed significant improvements in my fistula maturation rates without any complications or patient complaints, signalling a transformative advancement in our practice and a leap forward for patient care.”
Blessios – New York, USA
“When I first heard about VasQ, I was sceptical it would improve my outcomes. I have observed how strict patient selection based on arterial diameter and flow as well as an aggressive follow-up protocol can yield high rates of functional success but was curious if the device had the potential to standardise my surgical technique.17,18 The VasQ US Pivotal Study also interested me in the device’s potential to prevent longer term complications like aneurysm formation. In my first cases, I could clearly see and feel the difference with VasQ in place. The externally supported fistulas simply felt better and gave me more confidence in their potential for success with a reduced need for secondary procedures. I am still in the early stages of assessing this technology, but so far the results tell me that this device will likely be the standard for my fistula creations going forward.”
Gibbs – Portsmouth, UK
“I became interested in VasQ when I saw the outcome improvements being reported from a variety of vascular access experts at meetings like the Charing Cross (CX) symposium and Vascular Access Society (VAS) annual congress. As the lead surgeon for vascular access surgery at Portsmouth Hospital, I see many high-risk patients with low probability of fistula success. Having incorporated VasQ into my practice, I would say it has led to more consistency in fistula creation and improved outcomes for my patient population, even when implanted by less experienced surgeons in my centre. Traditionally high-risk patients now have a fighting chance at a sustained, well-functioning fistula with VasQ in place—it’s a game changer for the vascular access community!”
Enming & Li – Novena, Singapore
“Our hospital, Tan Tock Seng, has been a pioneer of the VasQ device in Singapore and among the first to implant the device in the country. My colleagues and my interest in VasQ stemmed from its engineering and how it was designed to impact the haemodynamics of the fistula. Our initial results are promising in an Asian population with smaller vessel diameter, with no primary failures or infections so far, and a mean time to cannulation of 68 days. Additionally, all fistulas were considered radiologically mature at one month with only 20% requiring balloon assisted maturation compared to a baseline of 30–40% of fistulas requiring balloon assisted maturation. Implanting VasQ to support our fistulas has helped to reduce the need for balloon assisted maturation in our practice whilst reducing the rate of primary failures.”
VasQ represents more than just a medical device—it embodies a promise of hope and progress for dialysis patients worldwide and the vascular surgeons, nephrologists, and other integral healthcare workers who strive to provide the best possible care to end-stage renal disease (ESRD) patients. With its innovative design and proven efficacy, VasQ stands as a testament to the power of innovation in medicine, offering a glimpse into a future where improved patient outcomes and enhanced surgical techniques are not just aspirations, but tangible realities.
*VasQ is intended for use as an external support for upper extremity arteriovenous fistulas created for vascular access by means of vascular surgery.
References
- Dember et al. JAMA. 2008;299(18):2164-2171
- Huijbregts et al. Clin J Am Soc. Nephrol. 2008; 3:14-719
- Masengu A, et al. Clin Kidney J. 2016 Feb;9(1):142-7
- Allon et al. Am J Kidney Dis. 2018;71(5):677-689
- Irish et al. JAMA Intern Med. 2017;177(2):184-193
- Bleyer et al. J Vasc Surg. 2019; 69:507-15
- Peden et al. J Vasc Access. 2022 Mar;23(2):265-274.
- Karydis et al. Am J Kidney Dis. 2019;75(1):45-53
- Chemla et al. J Vasc Access. 2016; 17(3):243-248
- Shahverdyan et al. Semin Dial. 2023;36(2):147-154.
- Leonardi et al. J Vasc Access. 2021 Jul;22(4):658-665.
- Publications in progress. Data on file with Laminate.
- Swiecka, Zippel, Storck GMS 2021
- Shahverdyan et al. J Vasc Surg. 2022 Jan;75(1):248-254
- Karydis, Mallios, Mestres, Matoussevitch VAS 2021
- Dillavou, Ozaki, Hentschel, Lucas VEITH 2021
- Blessios et al. J Vasc Access. 2020 Jul;21(4):434-439.
- Blessios et al. J Vasc Access. 2016 Mar-Apr;17(2):124-30