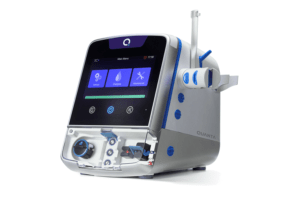
Quanta Dialysis Technologies has announced that it has completed enrolment of its Home Run study for at-home haemodialysis. The Home Run study is a prospective, multi-centre, open-label trial to assess the efficacy and safety of the Quanta Dialysis System for home haemodialysis. Results are expected to be announced in the second half of 2023.
“People with end-stage kidney disease (ESKD) on haemodialysis are currently limited as to options available. Although frequent home haemodialysis is proven to deliver superior outcomes, for many, in-centre dialysis treatment three times a week is the only viable option,” said Quanta chief medical officer, Paul Komenda. “We hope that FDA clearance of an additional home haemodialysis device will contribute to more patients having access to regular care in their home environment. Enrolment completion is the first step forward in a potential new alternative for people with this life-threatening disease.”
The Home Run study, a press release details, is establishing non-inferiority in safety and efficacy when the Quanta Dialysis System is used in the self-care home environment with a care partner compared to a haemodialysis facility. Following enrolment, study participants begin haemodialysis treatments using the system on a prescription of four-hour treatments, three times per week for a minimum of four weeks. During this time, the press release adds, “both patients and their caregivers will undergo extensive training and competency sign off on all aspects of safely administering haemodialysis treatment in the home”. Upon completion of training and a one-week transition period, participants will perform home treatment four times per week for three-and-one-half hours per treatment for eight weeks.











