Tag: ESRD
Quanta dialysis system receives US FDA clearance for home haemodialysis
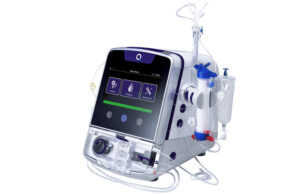
Quanta Dialysis Technologies has confirmed the US Food and Drug Administration (FDA) has given 510(k) clearance for the use of its Quanta dialysis system...
ESRD strongly associated with adverse limb events in new study
A new investigation into the association between end-stage renal disease (ESRD) and major adverse limb events (MALEs) has found that not only is it...
Vascular Therapies initiates enrolment in ACCESS 2 clinical trial
Vascular Therapies has announced that the first patient in the ACCESS 2 clinical trial was enrolled by Nikhil Kansal at Harbor-UCLA Medical Center in Torrance, USA.
The...
Fresenius Medical Care North America introduces first single-use integrated bloodline to...
Fresenius Medical Care North America's (FMCNA) Renal Therapies Group recently announced the availability of the CombiSet Smartech. According to a company press release, this...
DaVita Clinical Research study indicates mRNA COVID-19 vaccines effective in dialysis...
Dialysis patients who received mRNA COVID-19 vaccines had a lower risk of COVID-19 diagnosis post-vaccination, and were less likely to be hospitalised or die...
Fresenius unveils intelligent home dialysis solutions at China International Import Expo
Fresenius Medical Care recently presented its advances in home dialysis solutions at the China International Import Expo (CIIE) in Shanghai, China. The company showcased...
PVI 2021: Global standardisation of guidelines and patient-first approach to kidney...
In the future, a global approach to help standardise clinical practice guidelines for kidney disease may be the ideal way forward. This was the...
CMS takes “decisive steps” to reduce healthcare disparities among kidney disease...
The Centers for Medicare & Medicaid Services (CMS) is taking action to close health equity gaps by providing Medicare patients living with end-stage renal...
Strive Health unveils dedicated platform to help nephrologists deliver holistic, patient-centred...
Strive Health has announced Strive Care Partners (SCP)—a dedicated platform that enables nephrologists to share and succeed in global risk contracts with payors, according...
ImpediMed gains FDA Breakthrough Device designation for digital renal failure platform
ImpediMed, an Australia-based medical technology company using bioimpedance spectroscopy (BIS) to generate powerful data to improve patient health, has announced that the Sozo digital...
Fresenius and Cigna expand efforts to help people living with kidney...
Fresenius Medical Care North America (FMCNA) and Cigna have announced an expansion of their national effort to improve health outcomes and lower the cost...
Fist Assist device achieves significant perforator vein dilation for improved endoAVF...
Fist Assist Devices has announced that data associated with the p-FACT cohort, a subset of the recently completed, non-significant risk Fist Assist clinical trial...
Global FACT trial reports significant superficial arm vein dilation with FA-1...
Fist Assist Devices has just announced completion of the FACT trial, which evaluated the use of an intermittent pneumatic compression device, model FA-1, to...
Monogram Health raises US$160 million in funding to accelerate “next-generation” kidney...
Monogram Health, a benefit management and care delivery company attempting to transform care for individuals with chronic kidney disease (CKD) and end-stage renal disease...
ERA-EDTA announces name change following late-breaking clinical data at 2021 congress
Fresh evidence on the usefulness of sonography to ease the burden of lung congestion in haemodialysis patients and “promising data” on a long-awaited targeted...
Baxter gains FDA clearance for next-generation AK 98 dialysis machine
American healthcare firm and global renal care innovator Baxter International has announced US Food and Drug Administration (FDA) 510(k) clearance of its next-generation Artificial...
XO Score scoring sheath platform gains CE mark to treat PAD...
Transit Scientific has announced CE mark clearance for its XO Score scoring sheath platform, allowing the technology to be used in the treatment of...
Vascular Therapies announces clinical results from phase 3 randomised multicentre clinical...
Vascular Therapies recently announced results from its phase 3 clinical trial in which Sirogen showed encouraging arteriovenous fistula (AVF) outcomes in elderly end-stage renal...
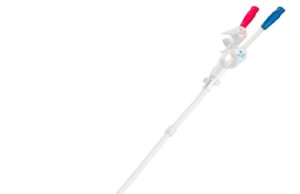
BD gains FDA 510(k) clearance for Pristine long-term haemodialysis catheter
American medical technology company BD (Becton, Dickinson and Company) has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Pristine...