 Fresh evidence on the usefulness of sonography to ease the burden of lung congestion in haemodialysis patients and “promising data” on a long-awaited targeted treatment for immunoglobin A nephropathy (IgAN) were among the highlights of several late-breaking presentations at the 58th European Renal Association-European Dialysis and Transplant Association Congress (ERA-EDTA, 5–8 June 2021, virtual). The role played by sodium-glucose co-transporter 2 (SGLT2) inhibitors, also known as “gliflozins”, in treating kidney diseases featured prominently during this session too.
Fresh evidence on the usefulness of sonography to ease the burden of lung congestion in haemodialysis patients and “promising data” on a long-awaited targeted treatment for immunoglobin A nephropathy (IgAN) were among the highlights of several late-breaking presentations at the 58th European Renal Association-European Dialysis and Transplant Association Congress (ERA-EDTA, 5–8 June 2021, virtual). The role played by sodium-glucose co-transporter 2 (SGLT2) inhibitors, also known as “gliflozins”, in treating kidney diseases featured prominently during this session too.
In addition to leading one of these presentations in the late-breaking session himself, ERA-EDTA president Christoph Wanner (University Hospital Würzburg, Würzburg, Germany) announced the shortening of the organisation’s name to simply the “ERA” soon after the event, following a “vast-majority” vote by its membership. “The reasoning behind this was that, in modern times, and with the communication we have today, it is simpler to use a shorter name for the association,” he said. “We communicate today, not only within the association, but also outside to other societies and members from different specialities.”

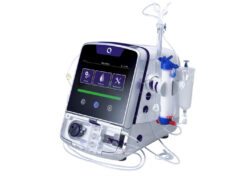
The results of a randomised multicentre study involving 363 patients—set up to assess whether ultrasound examinations, when compared to the standard of care, can help to detect lung congestion and guide decongestion in haemodialysis patients with high cardiovascular risk—were presented by Claudia Torino (National Research Council of Italy, Reggio Calabria, Italy) during a session devoted to late-breaking clinical abstracts on 6 June. The trial’s primary endpoints were mortality, heart attack and decompensated heart failure.
A
In the lung sonography group, the average number of B-lines in the ultrasound images—the measurement used to assess fluid accumulation in lung tissue—decreased from 15 to nine across the study, while this number increased from 16 to 30 in the control group. Secondary, post-hoc analyses showed “significantly less frequent” episodes of decompensated heart failure and cardiovascular events for the sonography group as well.
Torino stated that the study did, however, miss its primary composite endpoint, citing that there may not have been “sufficient time for the beneficial effect of the lung ultrasound-guided treatment to materialise”. And, despite this, she concluded: “Ultrasound examinations are available virtually everywhere in the hospital environment and do not take long to perform, so they can be deployed to diagnose and treat an ominous complication like lung congestion in haemodialysis patients.”
Another of the late-breaking presentations saw Wanner discuss a post-hoc analysis—using data from the EMPA-REG OUTCOME study—that looked at possible factors explaining how SGLT2 inhibitors slow the progression of chronic kidney disease, regardless of diabetes status. “According to the new study data, haematocrit is the most important mediator of the positive gliflozin effects, i.e., the main mechanism by which the SGLT2 inhibitors stabilise renal function, and improve the clinical outcome, correlates with the percentage increase in erythrocytes,” said Wanner. “These correlations now need to be investigated further—for example, to determine whether a role is played here by haemoconcentration due to increased urinary excretion induced by gliflozin, or by enhanced erythrocyte production.”

SGLT2 inhibitors remained a key topic in the session’s next presentation, with David Wheeler (University College London, London, UK) presenting a subgroup analysis of the DAPA-CKD study. The study previously showed a significant benefit in renal outcomes for chronic kidney disease patients with and without diabetes mellitus, following treatment with an SGLT2 inhibitor called dapagliflozin. The analysis presented by Wheeler built on this, however, by indicating that the beneficial effect of dapagliflozin extends to patients with focal segmental glomerulosclerosis (FSGS), as it reduces the progressive loss of renal function associated with this kidney disease type by half.
“SGLT2 inhibitors offer a promising new therapeutic option in the field of nephrology and are likely to be used more extensively in future, both in diabetic and non-diabetic kidney diseases,” he said. “Not only do these agents slow progression of kidney disease, but they also reduce the risk of cardiovascular diseases, which are important comorbidities in this patient population.”
Earlier on in the programme, interim analyses of iptacopan (Novartis)—a targeted treatment for the rare kidney disease IgAN—were presented by Jonathan Barratt (University of Leicester, Leicester, UK). The orally-administered drug was assessed in a randomised, double-blind placebo-controlled Phase II clinical trial involving a total of 112 patients, with the primary endpoint being the dose-response relationship in the improvement of proteinuria as a 24-hour urine protein:creatinine ratio.
The analysis showed a statistically significant dose-response effect of iptacopan versus placebo, with a 23% decrease in proteinuria under 200mg at 90 days, while renal function—assessed as estimated glomerular filtration rate (eGFR)—showed a trend to stabilisation, and measured biomarkers also improved. Side effects were mild (92%) to moderate (8%), and there were no serious adverse effects. In addition to stating that this is “the first study to report the safety and efficacy of targeted alternative complement pathway inhibition in IgAN patients”, Barratt added: “Iptacopan was also well-tolerated and resulted in inhibition of various biomarkers of alternative pathway activity, supporting its further evaluation in the ongoing Phase III APPLAUSE-IgAN trial.”

Elsewhere, results from the ACCOLADE study, which assessed the selective c5a receptor inhibitor avacopan’s (ChemoCentryx) ability to treat C3 glomerulopathy (C3G)—a rare condition that can progress to end-stage renal disease (ESRD)—were presented by Andrew Bomback (Columbia University, New York, USA). He reported improved C3HI (Histologic Index) disease activity and chronicity scores, as well as improvements in proteinuria, statistically-significant eGFR changes, and similar tolerability and safety profiles, between the trial’s avacopan and placebo groups at 26 weeks. “Targeted immunomodulation is already used to treat various diseases and will play an increasingly important role in the future if we want to treat C3G effectively, yet safely,” Bomback concluded.