As per an announcement on the company’s LinkedIn page, InnAVasc Medical has been granted US Food and Drug Administration (FDA) permission to commence the third phase of enrolment for its pivotal clinical study.
As per an announcement on the company’s LinkedIn page, InnAVasc Medical has been granted US Food and Drug Administration (FDA) permission to commence the third phase of enrolment for its pivotal clinical study.
“This is a big step forward to completion of the study,” the InnAVasc LinkedIn post states.
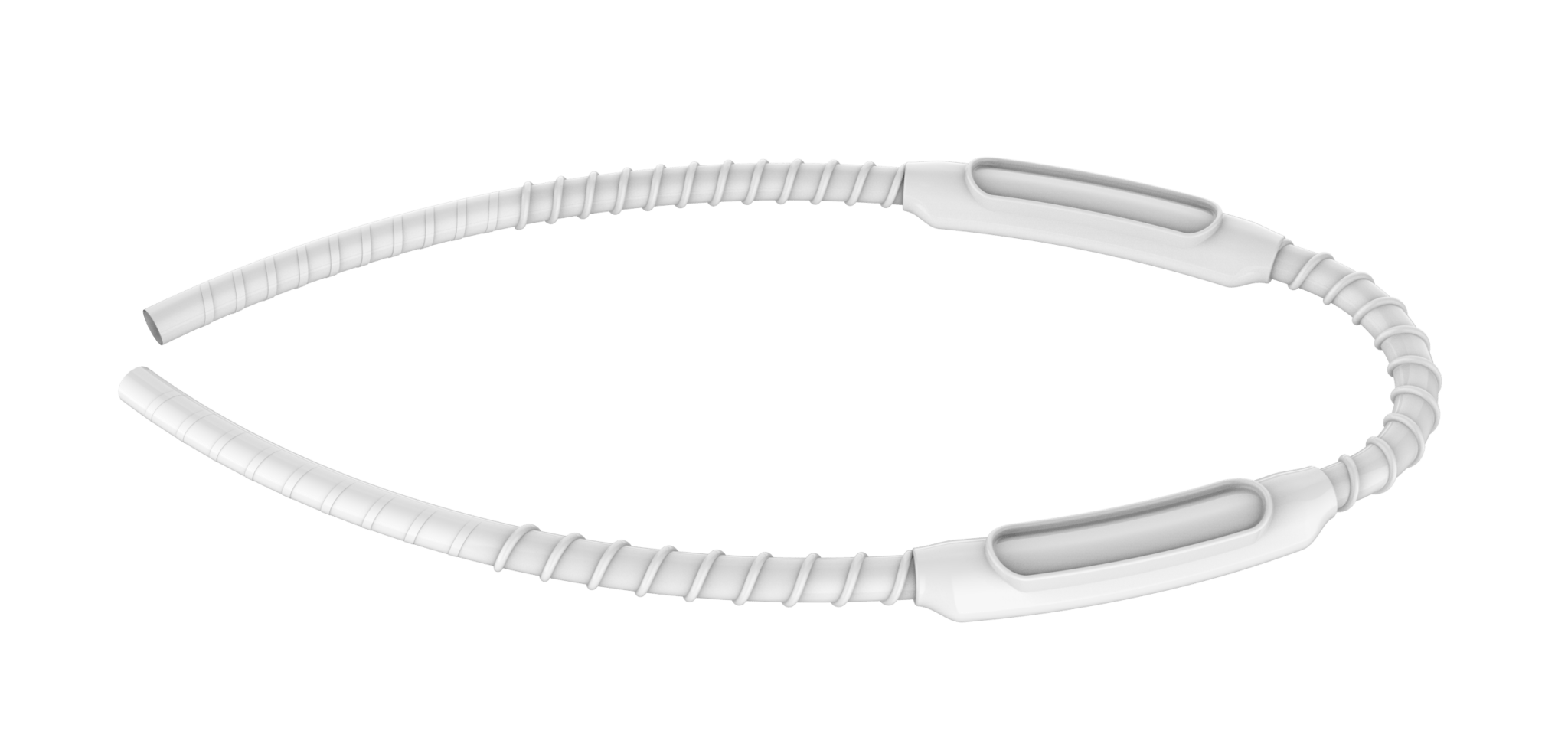
The pivotal study in question is intended to assess the safety and effectiveness of the company’s investigational haemodialysis graft—the InnAVasc Graft (IAVG)—which is designed to be used immediately post-implant.
The IAVG is intended to reduce needle-related injuries and complications and to be easier to locate under the skin, and—according to InnAVasc—it has the potential to facilitate more patients in the transition to home haemodialysis.
This news follows another announcement from late last year that the FDA had greenlit phase 2 of the InnAVasc pivotal clinical study.