Shanghai-based renal denervation (RDN) company Brattea has announced the successful completion of a Series B+ funding round worth more than US$20 million led by healthcare private equity fund Kuanping Capital.
According to a press release from Brattea, this is the largest ever investment event in China’s RDN sector. The company now plans to accelerate clinical trial progress to maintain its leading position in this market, and will attempt to quickly expand into several other promising indications including cancer pain and diabetes.
Brattea’s new investors following the completion of this Series B+ financing include Hengxu Capital, Pudong Science & Technology Co, and Brosmed, while investors from previous funding rounds include Northern Light Venture Capital, BioTrack Capital, and Sherpa Healthcare Partners.
Hongguang Cao, the company’s founder, chairman and CEO, said: “Since Medtronic acquired Ardian, many globally-renowned medical technology companies have invested into the RDN sector. Up to 2020, clinical trials began to show good results. Brattea is dedicated to innovating in the field of energy-based intervention devices and will keep pace with global leaders.”
According to Brattea, the prevalence of hypertension among adults in China is greater than 23%, representing a total of nearly 300 million patients, and about 50 million of these patients have refractory hypertension or chronic kidney disease (CKD) that can not be fully treated using drugs.
RDN technology has been verified by numerous clinical trials for its efficacy and safety in treating hypertension patients, the company also states, with results of the SPYRAL HTN-OFF MED (SPYRAL Pivotal) trial involving Medtronic’s Symplicity Spyral RDN system last year demonstrating an ability to “significantly” reduce blood pressure in the three months following surgery, without severe adverse events in the long term.
In addition, Medtronic’s real-world Global SYMPLICITY Registry indicates that systolic blood pressure could drop by 16.5mmHg in hypertensive patients—based on three-year follow-up data from more than 1,700 people.
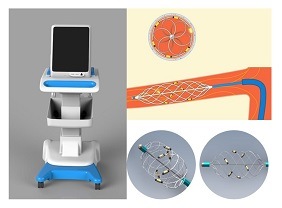
Brattea claims to have developed the world’s first basket-shaped, six-electrode ablation catheter with an innovative design and intelligent operating system, and has obtained a CE mark allowing its RDN products to be used in the European Union.
Brattea also states that RDN is one of the few sectors in which Chinese medical technology companies can keep up with global leaders, adding that its own products in this space have the potential to show clinical advantages compared to the Medtronic SPYRAL series.
Huihui Li, the founding partner and CEO of Kuanping Capital, said: “RDN will be the next blockbuster product due to innovative ideas, reliable products and proven clinical results. As a successful, serial entrepreneur, Cao’s sharp product perception, rich experience, and passion for innovation, will make him one of the unquestionable industry leaders. It is a great honour for Kuanping Capital to participate in this round of financing. Kuanping Capital will partner with this outstanding team to achieve great breakthroughs.”
Brattea says it is now enrolling a prospective, multicentre randomised controlled clinical trial to evaluate the safety and efficacy of its RDN products. Some 14 leading hospitals, including Chinese PLA Hospital, Ruijin Hospital, West China Hospital and the First Affiliated Hospital of Zhengzhou University, have already joined the trial, which is being led by Yujie Zhou—the deputy dean of Beijing Anzhen Hospital.











