Tag: renal denervation
First patients treated in US GPS study of Paradise renal denervation...
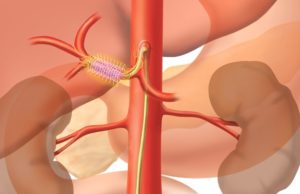
The first patients in the USA have been treated in Recor Medical’s Global Paradise System US Post Approval Study (US GPS), a real-world study...
SoniVie announces advancements in its renal denervation programme
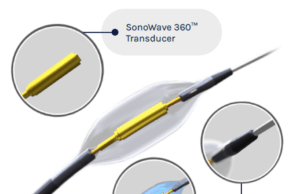
SoniVie, which has developed a novel Therapeutic Intra-Vascular Ultrasound System (TIVUS) to treat a variety of hypertensive disorders, presented at the EuroPCR 2024 conference...
Renal Interventions 12
Highlights
New trial evidence regarding the use of semaglutide for patients with type 2 diabetes and chronic kidney disease from the 61st European Renal...
Is renal denervation out of retirement?
“It’s rare to see a single trial kill something that was greeted with enthusiasm, so the question is why is renal denervation alive again?...
Access monitoring and vessel management spotlighted in Renal Interventions Controversies session
During the Renal Interventions Controversies programme that took place on the first day of the recent Charing Cross (CX) International Symposium (23–25 April, London,...
Renal denervation: Bridging specialty boundaries with better collaborations
Wei Li (Syracuse VA Medical Center, SUNY Upstate Medical University Syracuse, USA, and Texas Tech University School of Medicine, Lubbock, USA) and Aiyu Zhao...
Renal Interventions 11 – EU
Highlights
New trial evidence about the benefits of preoperative forearm exercises for vein diameter from Koen van der Bogt (Leiden, The Netherlands)
The importance...
Renal Interventions 11 – US
Highlights
New trial evidence about the benefits of preoperative forearm exercises for vein diameter from Koen van der Bogt (Leiden, The Netherlands)
The importance...
Renal Interventions’ top 10 stories of February 2024
February was a month of trials beginning enrolment, novel technologies, and treatment protocols, with Gianmarco Falcone emphasising the need for a full protocol and...
Study of TIVUS renal denervation system for hypertension treatment completes enrolment
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the proprietary...
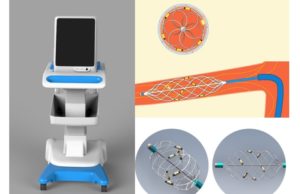
Renal denervation for hypertension shows promising results in RADIANCE II trial
A new trial of endovascular ultrasound-based renal denervation technology has found that it reduced blood pressure in hypertensive patients at two months. The RADIANCE...
Renal Interventions’ top 10 stories of February 2023
The 19th annual scientific meeting of the American Society of Diagnostic and Interventional Nephrology (ASDIN; 17–19 February, Orlando, USA) loomed large in February, with...
Results on ReCor Medical’s Paradise ultrasound renal denervation system published in...
ReCor Medical and its parent company, Otsuka Medical Devices, recently announced that primary endpoint results from the RADIANCE II pivotal trial were published in...
ESC and EAPCI publish renal denervation consensus statement
Renal denervation represents another treatment option in patients with uncontrolled resistant hypertension and may be used in selected patients deemed intolerant to antihypertensive drugs.
These...
Otsuka Medical Devices and ReCor Medical submit pre-market approval of Paradise...
Otsuka Medical Devices and ReCor Medical, a subsidiary of Otsuka, announce the filing of the pre-market approval (PMA) application to the US Food and Drug...
ReCor Medical announces consistent reduction of blood pressure in pooled analysis...
ReCor Medical and its parent company, Otsuka Medical Devices, announced consistent and significant blood pressure-lowering results across a range of patients with uncontrolled hypertension,...
SPYRAL HTN-ON MED study demonstrates meaningful clinical benefits consistent with other...
Medtronic has announced the six-month results from the full cohort of the SPYRAL HTN-ON MED clinical trial. The late-breaking data were presented at the...
TCT 2022 hears “important data” from renal denervation trials
Findings from the RADIANCE II randomised pivotal trial, presented at the 2022 Transcatheter Cardiovascular Therapeutics meeting (TCT, 16–19 September, Boston, USA), “confirm that ultrasound...
Ultrasound renal denervation meets primary efficacy endpoint in RADIANCE II study
ReCor Medical and Otsuka Medical Devices have announced that the RADIANCE II US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial...
SoniVie announces successful procedure with Tivus system on first patient enrolled...
SoniVie today announced that on 30 May this year the first patient was treated with its renal artery denervation Tivus therapeutic intravascular ultrasound technology, as...
SoniVie receives FDA IDE approval for pilot study to treat hypertension...
SoniVie recently announced that on 5 May 2022 the US Food and Drug Administration (FDA) granted investigational device exemption (IDE) approval for its REDUCED1...
SyMap Medical completes enrolment of SMART study for treatment of uncontrolled...
SyMap Medical has announced that it has completed enrolment of the SMART study, which will evaluate the safety and efficacy of targeted renal denervation...
SyMap Medical closes US$100 million financing round
SyMap Medical has announced the completion of a financing round that will be used to accelerate global clinical trials for the company’s renal mapping...
TCT 2021: Six-month RADIANCE-HTN TRIO results “open a window” for renal...
Six-month outcomes from the randomised RADIANCE-HTN TRIO trial, comparing endovascular ultrasound renal denervation to a sham procedure for treatment-resistant hypertension, demonstrate the additional effects...
Medtronic unveils data on hypertension treatment preferences, launches SPYRAL AFFIRM study
Medtronic today announced findings from a new study of patient preferences for the treatment of hypertension. The findings are set to be presented during...
SCAI releases proceedings from expert consensus roundtable on renal denervation treatment...
The Society for Cardiovascular Angiography and Interventions (SCAI) has released a proceedings document outlining the possible role of renal denervation (RDN) as a therapeutic...
Multi-organ denervation to combat cardiometabolic disease: A novel concept put to...
Following a successful proof-of-concept study, Gerard S Goh and Markus P Schlaich have embarked upon a first-in-human study to explore the safety and efficacy...
ACC.21: Ultrasound renal denervation shown to reduce blood pressure in resistant...
Ultrasound-based endovascular renal denervation can successfully reduce systolic blood pressure in patients with medication-resistant hypertension, as demonstrated by the results of a late-breaking clinical...
EuroPCR 2021: Late-breaking data demonstrate long-term benefits of Medtronic radiofrequency renal...
Medtronic today announced new clinical data from the Global SYMPLICITY registry (GSR) indicating that renal denervation with the Medtronic Symplicity renal denervation system was...
Metavention announces initiation of “groundbreaking” multi-organ denervation study
Metavention, a Minneapolis-headquartered medical device company, has announced the initiation of the MODUS study to treat patients with both type 2 diabetes and hypertension...
Renal denervation firm Brattea completes US$20 million Series B+ funding round
Shanghai-based renal denervation (RDN) company Brattea has announced the successful completion of a Series B+ funding round worth more than US$20 million led by...