This advertorial is sponsored by Merit Medical.
Maintaining an arteriovenous fistula/graft (AVF/AVG) is essential for long-term vascular access. However, over time, complications such as stenoses and occlusions can develop and may be life-threatening if not adequately addressed.
Endovascular solutions to overcome such complications have traditionally encompassed percutaneous transluminal angioplasty (PTA) and/or the insertion of bare metal stents. However, low patency rates often necessitate multiple reinterventions. Recently, a novel self-expanding endoprosthesis, the Wrapsody Cell-Impermeable Endoprosthesis (CIE; Merit Medical), was developed to improve access to the dialysis outflow circuit. Speaking to Renal Interventions, Robert Jones, an interventional radiologist at Queen Elizabeth Hospital Birmingham (Birmingham, UK), and Richard Bond, a vascular surgeon at Fiona Stanley Hospital (Perth, Australia) detail their experience with the device.
Bond was the first in the world to commercially implant Wrapsody CIE three years ago. “The first patient I remember well,” he says. “She had a left brachiocephalic fistula that had been put in place in 2014, and it had been working in the two years before I saw her in 2019. The main problem was that she was getting a lot of pain when the AVF was used for haemodialysis, and it was taking five to six hours to dialyse.” He adds that she “experienced bleeding from the fistula following dialysis.”

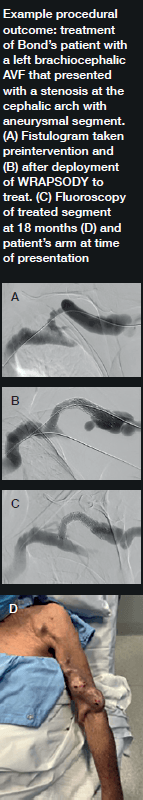
Bond explains that she was diagnosed with a particularly tight cephalic arch stenosis. Despite four previous treatment attempts by the radiology team rapid restenosis of her cephalic arch occurred each time. With her referral to Bond and the renal team, the initial treatment plan was to ligate the fistula. However, Bond opted for the Wrapsody CIE instead.
When asked why he chose Wrapsody CIE, he notes that, due to the anatomical anomaly present in this patient, no other endoprosthesis would have yielded a positive outcome. In addition, he indicates that the device’s design, specifically its tactile feel, made it a good treatment option. For Bond, the whole process was “very, very intuitive” and use of the device was “very straightforward”. As for the patient, he notes that she tolerated the procedure quite well: “The next day, the patient was achieving all of her dialysis goals, and her pain was resolved overnight. She is due for her three-year angiogram this month— she has not had any issues with dialysis.”

Jones echoes Bond’s suggestion that Wrapsody is straightforward to use. “It tracks very well through the vessel, over the wire and through the stenosis. On deployment the device remains stable, and the trigger delivery system is very responsive.”
On the device’s design, Jones highlights its unique tri-layer configuration, with its polytetrafluoroethylene (PTFE) inner layer, its cell-impermeable layer, and expanded PTFE (ePTFE) outer layer that allows it to be embedded into the vessel wall. He also cites its optimised radial strength and relative compression resistance.
“For me,” he says, “one of the most interesting design features is the softened end-rows. These are designed in a scalloped fashion with the aim of reducing stress at the interface between the edge of the stent and the normal adjacent vessel, which will hopefully reduce the incidence of edge stenosis—the Achilles’ heel of stent grafts.”
“It is also fairly unique in that there are larger diameter devices in its portfolio, including 14mm and 16mm devices, something not currently offered by other manufacturers. This will allow us to treat patients with central or thoracic vein stenosis, where larger diameter devices are often required for safe and effective treatment.”
Jones became more aware of the device and its potential after participating in its first-in-man study as part of the data monitoring committee. “My interest in the research related to this device stems from the concept that it has great potential for overcoming many of the shortcomings of stent grafts.” The first-in-man study yielded a target lesion primary patency rate of 84.6% and access circuit primary patency rate of 65.9% for Wrapsody at twelve months’ follow-up. These results, Jones says, told us that Wrapsody is “safe and effective for treating vascular access outflow stenosis”.1
He has since taken on the position of co-principal investigator in the WAVE study, a pivotal trial designed to enrol 244 patients with AVFs and 113 patients with AVGs across a maximum of 50 clinical sites globally. Patients in the AVF cohort are randomised to either the Wrapsody CIE or PTA, after an initial successful pre-dilatation. The planned enrolment of 357 patients is a “good number for a clinical trial of this nature”, Jones states.
Regarding the need for further research, Jones notes that WAVE is not the only study investigating the performance of this device and also underway is the WRAP global registry, aimed at expanding the safety and efficacy data on the device based on real-world experience. The registry has a 500-patient target globally, with enrolment having begun in June 2022. The prospective, multicentre, post-market observational study will also include peripheral cases in both AVF and AVG patients, as well as a thoracic central vein cohort. Patients will be followed for two years. The last patient follow-up visit is expected in 2026.
Since his first implantation three years ago, Bond has treated approximately 25 patients with the device. Yet he states that, in the beginning, he was less excited. “When I was first shown the device and its features explained to me, it was clearly a flexible but robust device,” he explains. However, regarding the cell-impermeable layer he “was a little sceptical” about its ability to stop in-device neointimal hyperplasia.
“What has really been obvious over the years, however, is that it really does do that,” he says. Reflecting on his experience with the Wrapsody CIE in his current practice, he concludes: “It has all of the strengths of any current covered stents, but it also has new features—and it is these that make it effective in places that other options have not been in the past.”
In particular, Bond believes the device’s clinical benefits will be particularly evident in the cephalic arch and central veins: “These are areas that are lacking in any good, suitable treatment option. There, it is really exciting, with a nice combination of flexibility and strength for the angles involved in those vessels.”
- Gilbert J, et al; First Clinical Results of the Merit WRAPSODY™ Cell-Impermeable Endoprosthesis for Treatment of Access Circuit Stenosis in Haemodialysis Patients CardioVascular and Interventional Radiology (2021). https://doi. org/10.1007/s00270-021-02953-8













