Laminate Medical Technologies has announced their flagship device, the VasQ External Vascular Support, has been cleared by the US Food and Drug Administration (FDA) for use to create arteriovenous fistulas (AVFs) for dialysis access. The device, designated by the FDA as a Breakthrough Technology, was cleared based on a de novo review of the 144 patient VasQ US pivotal study as well as a track record of safety and effectiveness of use in multiple studies from outside the USA.
As recently published in the Journal of Vascular Access, VasQ patients in the VasQ study met the primary endpoint of improved primary patency (freedom from intervention plus adequate flow for haemodialysis) at 6-months. No serious adverse event associated with the device was reported over the two year study. Additional analysis comparing VasQ patients against claims data for traditional AVFs created by the same surgeons in the study, reported statistically superior rates of functional success (confirmed use of the AVF for dialysis), and reduced need for additional procedures. The result was central venous catheters (CVC), the primary source of hospitalisation due to infection in dialysis patients, were able to be removed in 80% of the patients within the first year as compared to 62% of unsupported fistulas as reported by the National Institute of Health’s United States Renal Data System.
Ellen Dillavou, division chief of vascular surgery at WakeMed Hospital Systems, stated, “I, along with other study investigators who have used the device, are excited that we can now offer VasQ to our fistula patients. We believe this will give our patients a better chance to receive a functioning fistula with fewer additional procedures and will allow earlier removal of central venous catheters before they lead to serious infections.”
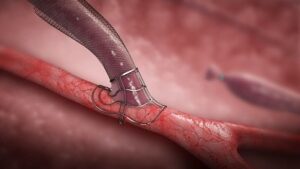
VasQ is a nitinol-based device implanted around the artery and vein during the surgical creation of an arteriovenous fistula. The device was designed to provide structural reinforcement of the mobilised vessels now freed from its native supporting tissue as well as guides a more stable arterial flow profile as it transitions into the vein. Magnetic resonance imaging as well as computational fluid dynamic models have supported the proposed mechanistic benefits of the VasQ design that have led to consistent beneficial clinical outcomes in multiple studies.
“We are excited to finally bring VasQ to the dialysis patient population in the USA,” said Laminate CEO Tammy Gilon. “We could not be more appreciative of our principal investigators as well as the global community of dialysis access physicians that saw the potential in VasQ and produced the wealth of data supporting the devices safety and effectiveness.”











