Metavention, a Minneapolis-headquartered medical device company, has announced the initiation of the MODUS study to treat patients with both type 2 diabetes and hypertension using a minimally-invasive, catheter-based approach.
Metavention, a Minneapolis-headquartered medical device company, has announced the initiation of the MODUS study to treat patients with both type 2 diabetes and hypertension using a minimally-invasive, catheter-based approach.
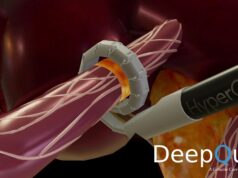
The first procedures in this study were successfully completed at The Alfred Hospital in Melbourne, Australia by interventional and diagnostic radiologist Gerard Goh, according to a statement from the US firm. The study procedures are being performed with Metavention’s novel, integrated iRF Denervation System.
“On behalf of the entire team at The Alfred, we are excited to be part of this groundbreaking study of the Metavention iRF Denervation System in patients with cardiometabolic syndrome,” said Goh. “The iRF denervation procedure has the potential to significantly alter how we look at treating patients suffering from cardiometabolic syndrome.”
The MODUS study is a prospective, three-arm, multicentre, randomised single-blind trial to evaluate the safety and performance of multi-organ sympathetic denervation in patients with type 2 diabetes and hypertension. Sympathetic nerve overactivity to key metabolic organs, such as the liver and kidneys, is associated with elevated blood pressure and uncontrolled glucose.
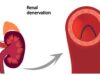
Multi-organ denervation is intended to inhibit this overactivity and, with a single treatment, improve control of hypertension and glucose. The MODUS study design uses randomisation to place subjects into one of three procedure groups—renal denervation for the kidneys, hepatic denervation for the liver, or multi-organ denervation for both the kidneys and liver.
Patients are currently being recruited for the MODUS study in Brisbane, Melbourne and Perth in Australia, following the study’s approval by the Human Research Ethics Committee.