
Jes S Lindholt, Thomas Emil Andersen and Martin Alm write together about TELEGRAFT, a prospective future graft solution that is being developed for the use in home haemodialysis and bypass surgery patients, what the issues are that they hope the graft solution can overcome, and how they intend the graft to do so.
With 10% of the worldwide population affected by chronic kidney disease and millions of deaths each year due to lack of access to dialysis units, there is a dire need for innovative and accessible devices which will help patients safely receive dialysis from home.
The unmet need
Artificial blood vessels have been used for more than 50 years when the patient does not have the necessary “spare parts”. However, very few improvements have been made to prevent the most frequent and serious complications, such as stenosing neointimal hyperplasia—scar tissue formation in the sutures between the graft and the native vessel slowing the blood flow, which causes them to close frequently, and problems with infection of the graft, which can occur even years after they have been inserted.
The problems are particularly frequent when they are used for dialysis, where up to 70% fail within a year. If they are used for bypass from the groin to the thigh, half close within five years, and they cannot be used for bypass of smaller blood vessels such as the coronary arteries on the heart.
By setting out to develop a graft solution for the most vulnerable patients—the dialysis patients—who can even use it for safe home dialysis without risking life-threatening bleeding, we are fairly certain that it can quickly also be used for bypasses in atherosclerosis; the potential therefore becomes enormous.
The innovative solution
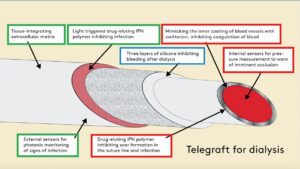 To combat this unmet need, we are collaborating to develop a special biomimetic and drug-eluting material for artificial blood vessels. The fundamental ideas are to use a special chemical to mimic a naturally occurring chemical found in the blood vessels of bears that is responsible for preventing the vessels from occluding whilst the animals hibernate during winter, as well as paclitaxel and antibiotics, which are intended for a slow internal release to prevent scar tissue formation in the suture line, and an external release on demand to combat impending bacterial infection, respectively.
To combat this unmet need, we are collaborating to develop a special biomimetic and drug-eluting material for artificial blood vessels. The fundamental ideas are to use a special chemical to mimic a naturally occurring chemical found in the blood vessels of bears that is responsible for preventing the vessels from occluding whilst the animals hibernate during winter, as well as paclitaxel and antibiotics, which are intended for a slow internal release to prevent scar tissue formation in the suture line, and an external release on demand to combat impending bacterial infection, respectively.
Blood poisoning arising from vascular prostheses is a frequent and life-threatening complication for dialysis patients. The new technology developed in the project makes it more difficult for the bacteria to attach to the material and thus prevents infections. In addition to that, the Swedish company VERIGRAFT contributes technology to improve integration with the surrounding tissue, thereby ensuring that the blood vessel grows quickly with its surroundings, and which, combined with a three-layer self-closing silicone wall, enables fast and safe dialysis—so safe that many dialysis patients will be able to look forward to using home dialysis, rather than spending several hours on transport to and staying at a dialysis clinic two to three times a week.
However, we do not believe that the complications can be completely ruled out. Due to this, we are attempting to equip the artificial blood vessels with two diagnostic tools, in the form of pressure gauges that can monitor the blood flow via telemonitoring—thereby warning of impending closure—as well as optical sensors that can pick up signs of incipient inflammation. Today, we usually only discover the complications when it is too late—but with telemonitoring, we get an early warning so that we can make corrections before the problems arise. It is a completely new approach which has enormous potential to reduce patients’ suffering and the costs to the healthcare system.
The (hopefully) upcoming graft is therefore aptly named TELEGRAFT.
Development of the graft started September 2022 and is planned to end February 2027 (54 months). The project is funded by Horizon Europe with a total budget of €5,298,235.75.
Disclosures: Jes S Lindholt is project coordinator and a professor of Vascular Surgery at Odense University Hospital (Odense, Denmark). Thomas Emil Andersen is an associate professor at the Department of Clinical Microbiology, University of Southern Denmark (Odense, Denmark). Martin Alm is a chemical engineer, head of polymers at Biomodics ApS (Rødovre, Denmark).
 Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the Health and Digital Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.












